The
FREEDOM
Study was a 26-week
maintenance,
multi-center,
uncontrolled,
open-label study of
newly
diagnosed or untreated
patients aged
≥12
years with partial-onset
seizures.1
A MULTICENTER, UNCONTROLLED, OPEN-LABEL STUDY IN PATIENTS AGED ≥ 12 YEARS2
4 mg Maintenance treated (mITT)

All patients presented with at least 1 of the following seizure types:1
•
Simple
partial
seizures
with or
without
motor
signs
(16%)
• Complex partial seizures (61%)
• Complex partial seizures with secondary generalization (64%)
The median baseline seizure frequency was 2 seizures per 12 weeks.1
ITT=intent-to-treat;
mITT=modified
intent-to-treat
Study design1

STUDY OBJECTIVE
The
objective was to
evaluate the
seizure-free
rate during a
26-week
Maintenance
Period.
The evaluation
criteria focused
on the efficacy
and safety of
FYCOMPA when
used as
monotherapy in
patients with
new-onset
partial-onset
seizures,
including
secondarily
generalized
seizures.1
The initial
approval of
FYCOMPA for
partial-onset
seizures, with
or without
secondary
generalization,
was based on 3
trials in
patients not
controlled with
1
to 3 concomitant
antiepileptic
drugs.2
These patients
had a mean
duration of
epilepsy of ~21
years and a
median baseline
seizure
frequency of 9
to 14 seizures
per 28 days.2
Based on an
extrapolation of
the data from
these 3
trials,
the indication
of FYCOMPA was
expanded to
include
monotherapy use
for
partial-onset
seizures.1
• 96% (70/73) of patients were newly diagnosed (mITT)1
•
The modified
intent-to-treat
(mITT) analysis
set (n=73) was a
subset of the
intent-to-treat
(ITT) analysis
set (N=89) that
entered the 4 mg
Maintenance
Period and
subsequently had
at least 1
post-dose
primary efficacy
measurement
• A Japanese and South Korean study1
The majority of newly diagnosed
or untreated
patients with partial-onset
seizures were convulsive
seizure free at 4 mg after 26
weeks of monotherapy1
4 MG SEIZURE-FREE RATES AT 26 WEEKS2
Convulsive Seizure Free at 4 mg
65%
OF PATIENTS
(n=31/48)
Partial-Onset Seizures
with Secondary
Generalization*
Seizure Free at 4 mg
63%
OF PATIENTS
(n=46/73)
Partial-Onset Seizures
21 out of 73 patients were not
controlled at 4 mg
and were titrated to 8
mg/day.1
LIMITATIONS
• The study was open-label and did not include a control arm.1
• Appropriate multiplicity adjustments were not applied.
• This information is descriptive.
*Among the 73 patients with partial-onset seizures who entered the 26-week 4 mg Maintenance Period of the Treatment Phase, 48 had secondarily generalized seizures at baseline.1
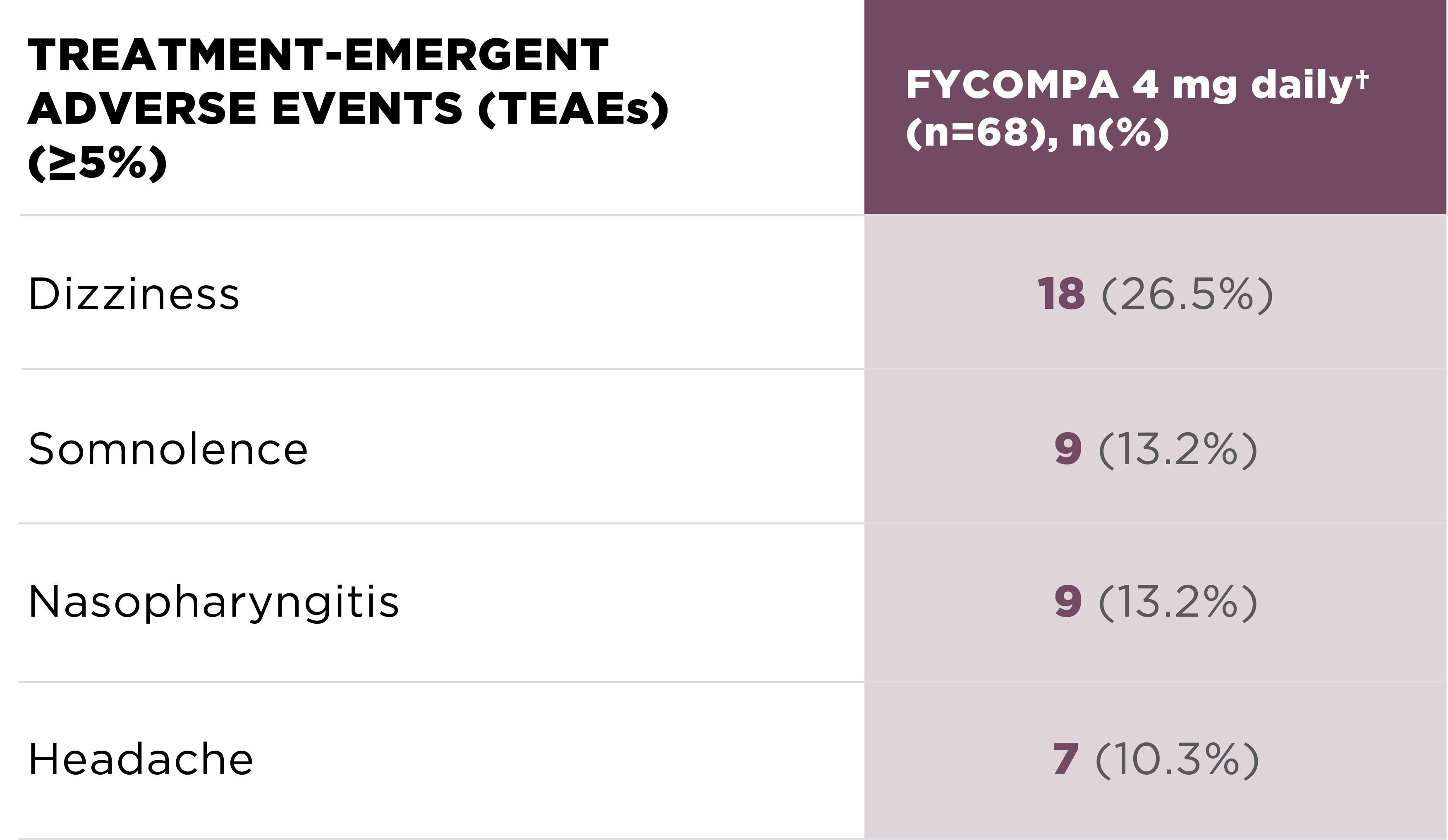
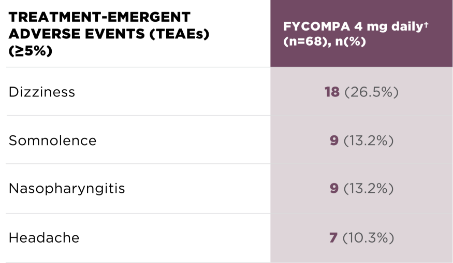
Adverse reactions at 26 weeks
during treatment
phase by 4 mg final dose
(n=68)1,*
ADVERSE REACTIONS THAT OCCURRED IN ≥5% OF PATIENTS TAKING FYCOMPA 4 MG
22 patients (24.7%) discontinued treatment during the 4 mg Treatment Phase1
•
Reasons: adverse
reaction (n=8; 9%),
subject choice (n=1;
1.1%), inadequate
therapeutic effect
(n=3; 3.4%), lost to
follow-up (n=2;
2.2%), withdrawal of
consent (n=5; 5.6%),
and other
(n=3;
3.4%)
Psychiatric disorders at 4 mg/day1,* (n=68)
• Irritability (n=2; 2.9%), affect lability (n=1; 1.5%), depression (n=1; 1.5%), and insomnia (n=1; 1.5%)
*Includes patients who completed
or
discontinued from the 4 mg
Treatment
Phase.
†Patients with a final dose of 2
mg were grouped
into 4 mg.
REFERENCES: 1. Data on file. Catalyst Pharmaceuticals Inc., Coral Gables, FL. 2. FYCOMPA US Prescribing Information. Coral Gables, FL: Catalyst Pharmaceuticals, Inc.
IMPORTANT SAFETY
INFORMATION AND
INDICATION
IMPORTANT SAFETY
INFORMATION
AND INDICATION
WARNING: SERIOUS PSYCHIATRIC AND BEHAVIORAL REACTIONS
•
Serious or
life-threatening
psychiatric and
behavioral
adverse
reactions
including
aggression,
hostility,
irritability,
anger, and
homicidal
ideation
and
threats have
been reported in
patients taking
FYCOMPA
• These reactions occurred in patients with and without prior psychiatric history, prior aggressive behavior, or concomitant use of medications associated with hostility and aggression
• Advise patients and caregivers to contact a healthcare provider immediately if any of these reactions or changes in mood, behavior, or personality that are not typical for the patient are observed while taking FYCOMPA or after discontinuing FYCOMPA
• Closely monitor patients particularly during the titration period and at higher doses
• FYCOMPA should be reduced if these symptoms occur and should be discontinued immediately if symptoms are severe or are worsening
SERIOUS PSYCHIATRIC AND BEHAVIORAL REACTIONS
In the partial-onset seizures clinical trials, hostility- and aggression-related adverse reactions occurred in 12% and 20% of patients randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 6% of patients in the placebo group. These effects were dose-related and generally appeared within the first 6 weeks of treatment, although new events continued to be observed through more than 37 weeks. These effects in FYCOMPA-treated patients led to dose reduction, interruption, and discontinuation more frequently than placebo-treated patients. Homicidal ideation and/or threat have also been reported postmarketing in patients treated with FYCOMPA. The combination of alcohol and FYCOMPA significantly worsened mood and increased anger. Patients taking FYCOMPA should avoid the use of alcohol. Patients, their caregivers, and families should be informed that FYCOMPA may increase the risk of psychiatric events. Patients should be monitored during treatment and for at least one month after the last dose of FYCOMPA, and especially when taking higher doses and during the initial few weeks of drug therapy (titration period) or at other times of dose increases. Similar serious psychiatric and behavioral events were observed in the primary generalized tonic-clonic (PGTC) seizure clinical trial.
SUICIDAL BEHAVIOR AND IDEATION
Antiepileptic drugs (AEDs), including FYCOMPA, increase the risk of suicidal thoughts or behavior in patients. Anyone considering prescribing FYCOMPA or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Patients, their caregivers, and families should be informed of the risk and advised to monitor and immediately report the emergence or worsening of depression, suicidal thoughts or behavior, thoughts about self-harm and/or any unusual changes in mood or behavior. Should suicidal thoughts and behavior emerge during treatment, consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
DIZZINESS AND GAIT DISTURBANCE
FYCOMPA caused dose-related increases in events related to dizziness and disturbance in gait or coordination. Dizziness and vertigo were reported in 35% and 47% of patients in the partial-onset seizure trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 10% of placebo-treated patients. Gait disturbance related events were reported in 12% and 16% of patients in the partial-onset seizure clinical trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 2% of placebo-treated patients. These adverse reactions occurred mostly during the titration phase. These adverse reactions were also observed in the PGTC seizure clinical trial.
SOMNOLENCE AND FATIGUE
FYCOMPA caused dose-dependent increases in somnolence and fatigue-related events. Somnolence was reported in 16% and 18% of patients in the partial-onset seizure trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 7% of placebo-treated patients. Fatigue-related events were reported in 12% and 15% of patients in the partial-onset seizure trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 5% of placebo-treated patients. These adverse reactions occurred mostly during the titration phase. These adverse reactions were also observed in the PGTC seizure clinical trial. Patients should be advised against engaging in hazardous activities requiring mental alertness, such as operating motor vehicles or dangerous machinery, until the effect of FYCOMPA is known. Patients should be carefully observed for signs of central nervous system (CNS) depression when FYCOMPA is used with other drugs with sedative properties because of potential additive effects.
FALLS
Falls were reported in 5% and 10% of patients in the partial-onset seizure clinical trials randomized to receive FYCOMPA at doses of 8 mg and 12 mg per day, respectively, compared to 3% of placebo-treated patients.
DRUG REACTION WITH EOSINOPHILIA AND SYSTEMIC SYMPTOMS (DRESS)
DRESS, also known as multiorgan hypersensitivity, has been reported in patients taking AEDs, including FYCOMPA. DRESS may be fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement. If signs or symptoms are present, immediately evaluate the patient and discontinue FYCOMPA if an alternative etiology for signs or symptoms cannot be established.
WITHDRAWAL OF AEDs
A gradual withdrawal is generally recommended with AEDs to minimize the potential of increased seizure frequency, but if withdrawal is a response to adverse events, prompt withdrawal can be considered.
MOST COMMON ADVERSE REACTIONS
The most common adverse reactions in patients aged 12 years and older receiving FYCOMPA (≥5% and ≥1% higher than placebo) include dizziness, somnolence, fatigue, irritability, falls, nausea, weight gain, vertigo, ataxia, headache, vomiting, contusion, abdominal pain, and anxiety. Adverse reactions in patients 4 to <12 years were generally similar to patients aged 12 years and older.
DRUG INTERACTIONS
FYCOMPA may decrease the efficacy of contraceptives containing levonorgestrel. Plasma levels of perampanel were decreased when administered with known moderate and strong CYP3A4 inducers, including, carbamazepine, phenytoin, or oxcarbazepine. Multiple dosing of FYCOMPA 12 mg per day enhanced the effects of alcohol on vigilance and alertness, and increased levels of anger, confusion, and depression. These effects may also be seen when FYCOMPA is used in combination with other CNS depressants.
PREGNANCY AND LACTATION
Physicians are advised to recommend that pregnant patients taking FYCOMPA enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. Caution should be exercised when FYCOMPA is administered to pregnant or nursing women as there are no adequate data on the developmental risk associated with use in pregnant women, and no data on the presence of perampanel in human milk, the effects on the breastfed child, or the effects of the drug on milk production.
HEPATIC AND RENAL IMPAIRMENT
Use in patients with severe hepatic or severe renal impairment is not recommended. Dosage adjustments are recommended in patients with mild or moderate hepatic impairment. Use with caution in patients with moderate renal impairment.
DRUG ABUSE AND DEPENDENCE
FYCOMPA is a Schedule III controlled substance and has the potential to be abused and lead to drug dependence and withdrawal symptoms including anxiety, nervousness, irritability, fatigue, asthenia, mood swings, and insomnia.
INDICATION
FYCOMPA® (perampanel) is indicated in patients with epilepsy aged 4 years and older for partial-onset seizures (POS) with or without secondarily generalized seizures and adjunctive therapy for patients aged 12 years and older for primary generalized tonic-clonic (PGTC) seizures.
View the Prescribing Information



